药政注册
Regulatory Affairs
汉维拥有一支有10年以上国际药政注册经验的药政注册团队,经过多年努力,迄今为止汉维共获得12项国家新药证书。
新兽药注册申报需要对产品进行系统的研究,包括处方筛选、工艺优化、生产验证,临床研究等,以确保产品质量及安全性和有效性。凭借12个新兽药成功的注册经验,汉维能精准的把握各类产品的注册要求,并与相关部门建立了良好的关系,因此可以在较短的时间获得产品注册。
此外,汉维也承接国际注册业务,为国外客户进入中国市场给出进口政策和市场分析,并帮助其制定注册计划,完成进口注册。
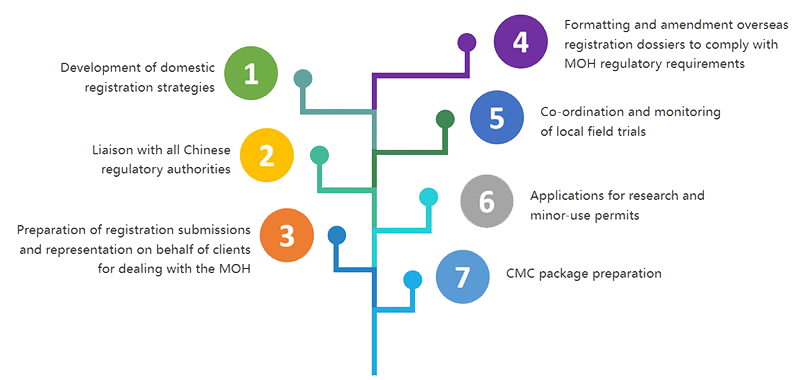
Proficient in international and domestic drug registrations for more than 10 years, Hanvet’s Regulatory Affairs team has obtained 8 New Drug Certificates in China, making Hanvet holder of the most First Generic Drug Certificates in China for companion animal healthcare products.
First Generic Drug application in China requires systematic know-how in formulation selection, process optimization, manufacturing validation, clinical studies, etc. to ensure quality, safety and potency.
Hanvet holds rich experience in drug registration and keeps close communications with authorities in China. Besides registration for their own products. Hanvet also offers registration services for overseas clients as well as CMC pacakge preparation.